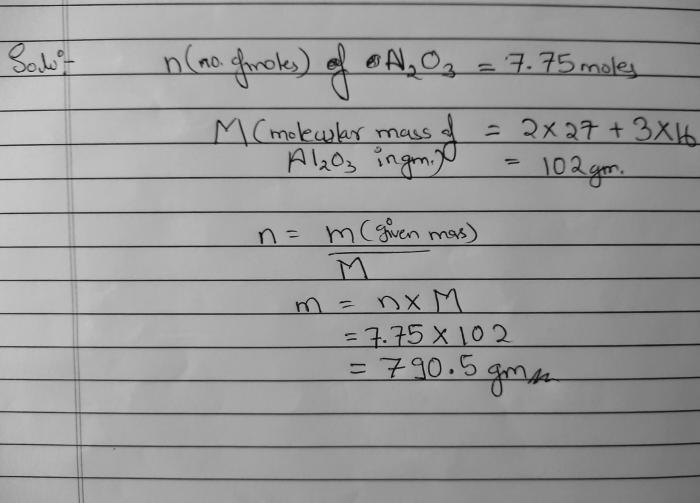Calculate the number of moles in 68.8 grams of al2o3 – In the realm of chemistry, understanding the concept of moles is crucial for quantifying the amount of a substance present. The mole, a fundamental unit of measurement, provides a bridge between the macroscopic and microscopic worlds, enabling us to determine the number of particles in a given sample.
This exploration delves into the calculation of moles in 68.8 grams of aluminum oxide (Al2O3), a compound commonly used in various industrial and scientific applications.
To embark on this calculation, we must first establish the molar mass of Al2O3, a value that represents the mass of one mole of the compound. Armed with this knowledge, we can then employ the formula moles = grams / molar mass to determine the number of moles present in the given mass of Al2O3.
Calculating the Number of Moles in 68.8 Grams of Al2O3: Calculate The Number Of Moles In 68.8 Grams Of Al2o3
In chemistry, the mole is a fundamental unit of measurement that quantifies the amount of a substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). Knowing the molar mass of a compound allows us to convert between its mass and the number of moles present.
Calculating Molar Mass of Al2O3
Aluminum oxide (Al2O3) is a chemical compound composed of aluminum and oxygen. To calculate its molar mass, we need to consider the atomic masses of aluminum and oxygen from the periodic table:
- Aluminum (Al): 26.98 g/mol
- Oxygen (O): 16.00 g/mol
The molar mass of Al2O3 is the sum of the atomic masses of two aluminum atoms and three oxygen atoms:
Molar mass of Al2O3 = (2 × 26.98 g/mol) + (3 × 16.00 g/mol) = 101.96 g/mol
Converting Grams to Moles
To convert grams of a substance to moles, we use the following formula:
Moles = Grams / Molar mass
Using this formula, we can calculate the number of moles in 68.8 grams of Al2O3:
Moles = 68.8 g / 101.96 g/mol = 0.675 moles
Reporting the Result, Calculate the number of moles in 68.8 grams of al2o3
Therefore, 68.8 grams of Al2O3 contains 0.675 moles of the compound. This result provides valuable information about the amount of substance present, allowing us to make further calculations or comparisons related to the chemical reactions or properties of Al2O3.
FAQ Guide
What is the significance of calculating the number of moles in a substance?
Determining the number of moles allows us to quantify the amount of a substance present, enabling accurate calculations in chemical reactions and other quantitative analyses.
How does the molar mass of a compound influence the calculation of moles?
The molar mass, representing the mass of one mole of a compound, is a critical factor in converting grams to moles. It provides the conversion factor necessary for this calculation.